
全部
▼
搜索
熱搜:
位置:中冶有色 >
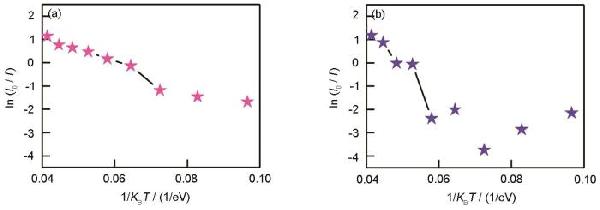
> 氧化石墨烯的變溫發(fā)光
 992
編輯:中冶有色技術(shù)網(wǎng)
來源:李福祿,韓春淼,高嘉望,蔣健,許卉,李冰
992
編輯:中冶有色技術(shù)網(wǎng)
來源:李福祿,韓春淼,高嘉望,蔣健,許卉,李冰
 分享 0
分享 0
 舉報 0
舉報 0
 收藏 0
收藏 0
 反對 0
反對 0
 點贊 0
點贊 0

 中冶有色技術(shù)平臺
中冶有色技術(shù)平臺 2025年03月21日 ~ 23日
2025年03月21日 ~ 23日  2025年03月21日 ~ 23日
2025年03月21日 ~ 23日  2025年03月25日 ~ 27日
2025年03月25日 ~ 27日  2025年03月29日 ~ 31日
2025年03月29日 ~ 31日  2025年04月24日 ~ 27日
2025年04月24日 ~ 27日 
